The rotator cuff is a group of tendons and muscles about the shoulder that provides stabilization and movement to the shoulder joint. These tendons, however, are prone to degeneration and rupture due to aging, injury, or overuse. Rotator cuff tears can cause significant shoulder pain and dysfunction, and when the entire thickness of the tendon is torn, surgery is usually the preferred solution to relieve pain and restore strength. The operation consists of repairing the torn tendons back to the native bone. Rotator cuff repair is one of the most common orthopedic procedures in the United States, with over 250,000 repairs performed each year.
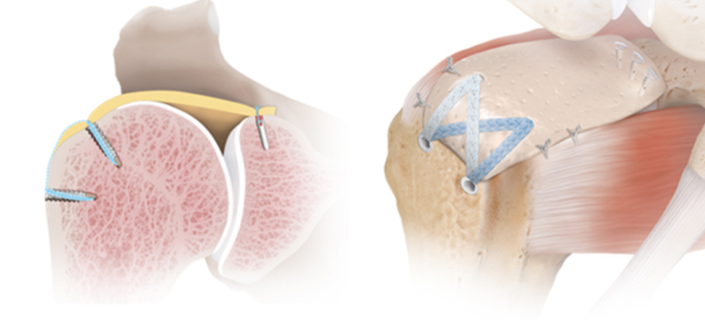
The management of rotator cuff tears that are irreparable despite advanced mobilization techniques has always represented a challenge in treating orthopedic surgeons. Historically, when non-operative treatment options failed to provide symptoms relief, surgical solutions included debridement of the torn tendons and sacrificing the biceps tendon. These options, however, yielded inconsistent outcomes. With the understanding that restoration of the transverse force couple, partial rotator cuff repairs gained popularity in a selected subset of patients. Following the same path as previous operative strategies, subsequent clinical studies on partial rotator cuff repairs with longer follow-up demonstrated mixed outcomes and pain relief, especially in the older population and for patients with limited active range of motion and strength
In the late 1980’s muscle tendon transfers were offered as a new solution to the management of irreparable rotator cuff tears. Unfortunately, the positive outcomes achieved by developers of this technique were not reproduced by most of other series by other investigators. With the Food and Drug Agency (FDA) approval of the Reverse Total Shoulder Replacement, some authors reported the use of this implant as a viable treatment option in this patient population, even in the absence of glenohumeral arthritis.
In 2012, Mihata et al., in Japan, introduced tensor fascia lata (TFL) autograft for the superior capsular reconstruction (SCR) as a joint preservation solution for irreparable and massive rotator cuff tears. A series of cadaveric biomechanical studies subsequently corroborated this technique. Since then, there has been an increasing interest in this technique by both orthopedic surgeons and industry for treating pre-arthritic patients with irreparable posterior superior rotator cuff tears.
The purpose of this text is to summarize the current literature regarding SCR for the treatment of massive and irreparable RCTs with specific attention to the anatomy, biomechanics and indications.
Anatomy:
In 1992, Clark et al. published a macroscopic and microscopic anatomical study of 32 intact cadaveric rotator cuffs. The authors demonstrate that the rotator cuff has five distinct histologic layers with layers two and three, consisting of the coracohumeral ligament (CHL) and the deepest layer consisting of a distinct superior joint capsule. Gohlke et al. followed this investigation with another microscopic study confirming the finding of a distinct superior capsular layer with an intricate pattern of collagen fiber cross-linking as opposed to the simpler radial collagen fiber pattern in the posterior capsule. It was then made clear that the glenohumeral superior capsule consisted of a distinct and highly organized layer that is intimate but separate from the articular fibers of the posterior superior rotator cuff. Nimura et al. went further and were able to dissect the rotator cuff and peel it away from the superior capsule to carefully study the anatomic footprint of both the capsule and tendon insertions in 12 shoulders of six matched cadaveric specimens (Figure 1). The authors demonstrated that the supraspinatus presented a triangular-shaped footprint, with the base lying along the articular surface on the most anterior superior facet of the greater tuberosity with an average width of 3.5 mm. At this location the width the superior capsule along the most anterior margin of the greater tuberosity was found to occupy a larger area of 5.6 mm. Most anatomic textbooks describe the infraspinatus as inserting in the middle facet of the greater tuberosity; however, Nimura et al. describe the footprint as occupying all of the middle facet and the posterior superior facet; additionally, the most anterior humeral insertion almost reached the anterior margin of the highest impression of the greater tuberosity. At the point of the maximum width of the infraspinatus, the average width of the capsular attachment was 5.4 mm, and the infraspinatus trapezoidal-shaped footprint was 9.7 mm. At the border between the infraspinatus and the teres minor, the average width of the articular capsule increased to 9.1 mm. Previous authors reported a wider rotator cuff footprint but did not independently dissect the capsule away from the rotator cuff.
In a follow-up study, Momma et al. corroborated Nimura et al. finding of a broad and robust superior capsular insertion. Additionally, the authors evaluated the capsule-labrum attachment on the glenoid and described a relatively constant width of the superior capsule from the 3’oclock (5.6 mm +/- 0.5 mm) to the 9’oclock position (6.1 mm +/- 0.3 mm). In summary, the cadaveric studies performed by the authors mentioned above complemented previous microscopic work demonstrating a distinct and robust superior capsular attachment on both the glenoid and humeral footprints.
Biomechanics:
Mihata et al. published their first cadaveric studies in 2012 on the superior instability of the glenohumeral joint with a simulated supraspinatus tear followed by restoration of the native superior translation with the reconstruction of the superior capsule. The study consisted of eight cadaveric specimens that were dissected free of all tissue except for the capsule, coracoacromial ligament, rotator cuff musculature, and the humeral insertion of the pectoralis major, latissimus dorsi, and deltoid muscles. The specimens were mounted in a shoulder jig, and the difference between the humeral head translation relative to the glenoid in the unloaded state compared to the state where the deltoid was tensioned was defined as the superior translation. Additionally, the authors measured subacromial contact pressures, range of motion, and glenohumeral joint compressive forces. The experiment called for measurements of the above parameters in various degrees of abduction and external rotation in the intact cuff state and supraspinatus tear state, followed by patch grafting, superior capsular reconstruction (SCR), and a combination of both graft patching and SCR. The authors found that the superior translation of the humerus significantly increased after the supraspinatus was excised and this was completely restored to the native state with a SCR and partially restored with patch grafting of the supraspinatus. The subacromial contact pressures, as measured by a pressure sensor, significantly increased with supraspinatus excision, and this was restored to the native state with both graft patching and SCR. The glenohumeral joint forces significantly decreased with excision of the supraspinatus and were not restored with graft patching, SCR, or a combination of both. Finally, a complete range of internal and external rotation motion was modestly decreased compared to the intact state in the SCR simulation but not in the graft patch simulation.
Mihata’s et al. published additional biomechanical studies with the same cadaveric shoulder jig set up looking at the effects of capsular continuity, graft thickness, and acromioplasty in conjunction SCR. In the capsular continuity study, the authors tested similar parameters of superior translation, subacromial contact pressures, glenohumeral joint forces, and range of motion. The experimental conditions included excision of the supraspinatus and SCR without side to side sutures, followed by SCR to infraspinatus sutures and SCR to both infraspinatus and subscapularis sutures. SCR without side-to-side sutures did not restore superior translation when the supraspinatus was excised; however, side-to-side sutures to the infraspinatus restored superior translation without an additional benefit of adding anterior side-to-side sutures. In opposition to what we would expect, this experiment found higher glenohumeral joint compressive forces in the SCR reconstruction compared to the intact state; additionally, there was no decrease in range of motion as opposed to the first biomechanical study.
Mihata et al. stressed the importance of acromioplasty when reconstructing the superior capsule clinically in order to avoid graft abrasion and potential failure in the technique description. This prompted the investigators to study the biomechanical effects of superior capsular reconstruction with and without acromioplasty. The major finding from this cadaveric study was that acromioplasty did not result in increased superior translation or subacromial contact pressures with the benefit of significantly decreasing the total contact area of the graft under the acromion. The final cadaveric experiment looking at optimization of superior capsular reconstruction was studying the effects of graft thickness (4 mm vs. 8 mm) and tensioning at 10o vs. 30o of abduction. The authors demonstrated complete restoration of superior migration to the intact state compared to the massive tear state in the 8 mm graft, with partial restoration in the 4 mm group. Tensioning the 8 mm graft at both 10o and 30o restored superior migration and subacromial peek contact pressures.
Graft options:
The original biomechanical and clinical data by Mihata et al. utilized autologous tensor fascia lata as the graft of choice, whereas dermal allograft is used in the United States literature (Figure 3). Mihata et al. investigated whether 3mm human dermal allograft would equally restore superior migration compared to a 6-8 mm fascia lata graft. The investigators found that the fascia lata graft completely restored the superior migration of the humeral head with the dermal allograft condition, only partially restoring superior migration. The authors postulated that the more flexible and thinner human dermal allograft might be inadequate in restoring superior migration compared to the stiffer and thicker fascia lata graft. Conversely, in a rabbit model of SCR, both fascia lata and acellular dermal graft demonstrated similar biomechanical and histological outcomes. More recently, other options of graft for reconstruction of the superior capsule have been proposed, including the biceps tendon or the patellar tendon.
Surgical Indications:
The surgical indications for a superior capsular reconstruction are an irreparable supraspinatus tear despite mobilization upon arthroscopy in the pre-arthritic shoulder (i.e., Hamada class III or less) in a physiologically active patient (Table 1). The mean age of patients in the available outcome studies was 60-65 years, as described in the outcomes section below. The presence of a subscapularis or infraspinatus tear should not be an absolute contraindication as long as these tendons are repaired in a tension-free manner at the time of surgery. The post-operative rehabilitation protocol may be more restrictive compared to a standard rotator cuff repair in, and the patient should be able and willing to comply with these restrictions. Contraindications include cervical nerve root or axillary nerve palsy, deltoid muscle dysfunction, and active infection. Pseudoparalysis is not an absolute contraindication to SCR in the appropriate patient. SCR showed to reverse pseudoparalysis both with fascia lata autograft or acellular dermal allograft
Table 1: Indications and contraindications to superior capsular reconstruction.
| Indications | Contraindications |
| Arthritic changes, Hamada stage > III | |
| Irreparable supraspinatus tear | Deltoid muscle dysfunction |
| Physiologically active patient, < 70 years | Axillary nerve palsy, cervical nerve root palsy |
| Ability to comply with postoperative rehabilitation | Irreparable infraspinatus or subscapularis tear |
| Active Infection |
Authors: Dr. Michael Wiater (MOS) and Dr. Leonardo Cavinnato (MOS) and Dr. Omar Khatib
For more information on the author and doctor’s credentials, please visit their profiles.
Leonardo Cavinatto MD
Beverly Hills Orthopaedic Surgeons
Visit Profile
J. Michael Wiater MD
Beverly Hills Orthopaedic
Visit Profile
